FDA Is One Step Away From Approving This 'Party Drug' to Treat PTSD

By:
The Food and Drug Administration authorized a series of large-scale clinical trials for the therapeutic use of MDMA — a psychoactive drug also known as Molly or ecstasy — to treat post-traumatic stress disorder (PTSD) on Tuesday. As a Phase 3 trial, it's success would represent the final step before the agency approved MDMA as a legal prescription drug.
 AP Images/Paul Faith - apimages.com
AP Images/Paul Faith - apimages.com
Though previous studies have offered compelling evidence of the drug's effectiveness at treating symptoms of PTSD, the Drug Enforcement Administration's decision to classify MDMA as a Schedule 1 drug, the most restrictive drug category under the federal government's list of banned substances, in 1985 has largely impeded research into its medical value for decades. The Phase 3 trial could be a turning point, allowing PTSD sufferers to receive the drug in a controlled, psychotherapy setting, administered by a doctor.
"I’m cautious but hopeful," Dr. Charles Marmar, who heads the psychiatry department at New York University’s Langone School of Medicine, told The New York Times. "If they can keep getting good results, it will be of great use. PTSD can be very hard to treat. Our best therapies right now don’t help 30 to 40 percent of people. So we need more options."
MDMA was banned after it was popularized as a "party drug" in the 1970s and 1980s, and experts agree that the drug carries some risk of abuse. That said, the perception of MDMA as an unsafe drug is due, in large part, to the fact that it's often combined with other substances such as amphetamines on the black market.
"Unless you are getting it from a psychiatrist in a legitimate clinical trial, at the present time, you can't guarantee what's in it, how much there is, or its safety, so I would say, as we have said in the past: Don't take it," Dr. Perry Kendall, the chief health officer of British Columbia, told CBC News.
The Multidisciplinary Association for Psychedelic Studies (MAPS), which has sponsored several clinical trials into the use of MDMA for PTSD treatment, explained that "pure MDMA has been proven sufficiently safe for human consumption when taken a limited number of times in moderate doses."
How would MDMA-assisted psychotherapy work?
 MAPS - maps.org
MAPS - maps.org
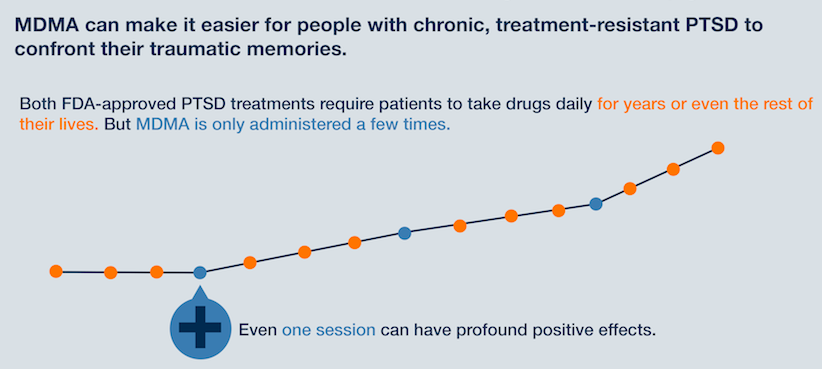
Clinical trials have found that MDMA, administered in moderate doses in tandem with conventional psychotherapy sessions, can alleviate symptoms of PTSD - such as: anxiety, agitation and insomnia - more effectively than when patients receive talk therapy alone. A Phase 2 trial from 2015 found that "83 percent of participants were cured of their PTSD" through MDMA-assisted psychotherapy, compared to "25 percent who were cured from talk therapy alone," according to NPR.
Researchers behind this latest phase are "so optimistic" about the progress of earlier trials that they've applied for "breakthrough therapy status," which would expedite the approval process, according to the New York Times. That means, if the Phase 3 trial is successful and earns FDA approval, the drug could be legal for specific medical uses as early as 2021.
